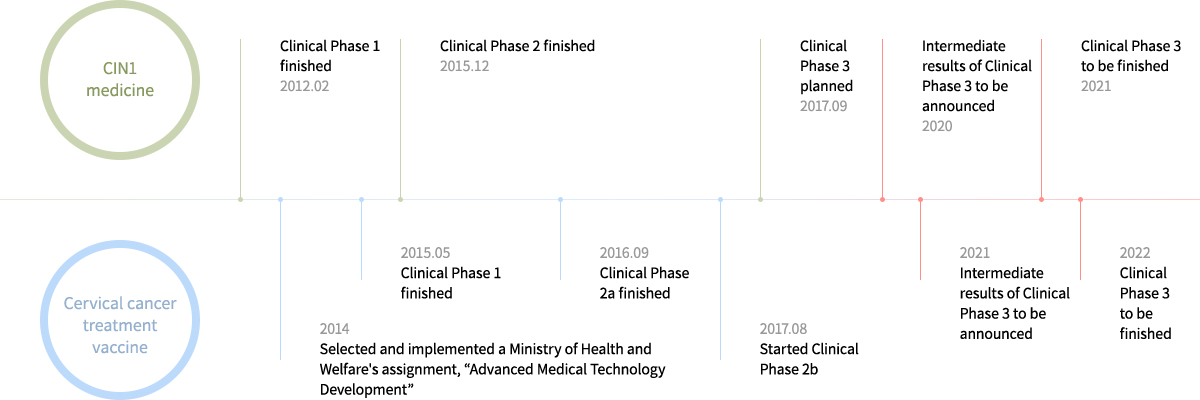
H. CIN1 medicine & M. cervical cancer treatment vaccine
Treatment or early examination of cervical diseases “First in its Class”
-
 Efficacy Test
Efficacy Test
-
 Toxicity Test
Toxicity Test
-
 Clinical Test
Clinical Test
| Platform technology | HumaMAX® | MucoMAX® | |
|---|---|---|---|
| Target disease | CIN1(BLS-H01) | CIN2/3(BLS-M07) | |
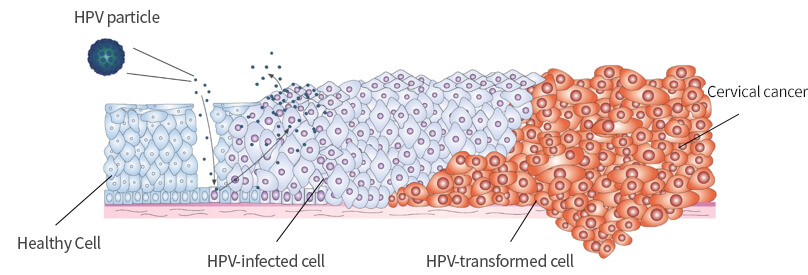
| Stage of disease progress | Normal/HPV infected | LSIL 1) | HSIL 2) |
 |
|||
| Treatment mechanism | Cells infected by the HPV Virus -> Prevents virus proliferation byusing BLS-H01 which has an antivirus effect |
Virus-infected cervical epithelial cells create abnormal cells as time passes,even leading to deformation of the intraepithelial tissues -> Disease treatment via inducing immunoreactions |
|
- LSIL : Low-grade squamous intraepithelial lesions
- HSIL: High-grade squamous intraepithelial lesions
Clinical Test
BLS-M07
Outcome
- Since August 2017, Clinical Phase 2b has been in progress with 16 testing agencies
- Goal of clinical tests : To prove the effectiveness of the medicine developed by BL, compared to the above drug
- Currently in a stage to check whether or not there is 75% treatment effectiveness, shown from Phase 1 and 2a, is meaningful from a statistical point of view
Market scale
- The global market estimated to be worth KRW 3.5 trillion, ‘First-in-class’ globally innovative new medicine
- Currently, no treatment options other than "conization" exist for stage 3 cervical cancer and, if a woman becomes pregnant after a conization procedure,
the likelihood of side effects such as premature birth and miscarriage as well as recurrences of cancer are high, which makes the development of an effective
medicine a pressing issue
Competitiveness
- The best “First-in-its-Class” medicine among a group of similar drugs
- Immunotherapy-based oral intestinal coating capsule : Convenience in administration and reduced psychological burden on the patients in the face of a treatment
- Mechanism to induce mucosal CTL immunity, which is the most ideal means by which to treat cervical mucosal disease : "Inducing antigen-specific immunity"
by absorbing antigens into a body through intestinal mucosa with lactic acid bacteria as the transporter
Strategy
- Achieve meaningful effects during Clinical Phase 2b and then proceed with global clinical trials
- About 250,000 people around the world die from cervical cancer every year
- CIN1 medicine [BLS-H01] and cervical cancer treatment vaccines [BLS-M07] are a Total Solution for HPV infections