H. Vaccine adjuvant
Open innovation among industry, government, and academia License-out strategies with global pharmaceutical companies
-
 Efficacy Test
Efficacy Test
-
 Toxicity Test
Toxicity Test
-
 Clinical Test
Clinical Test
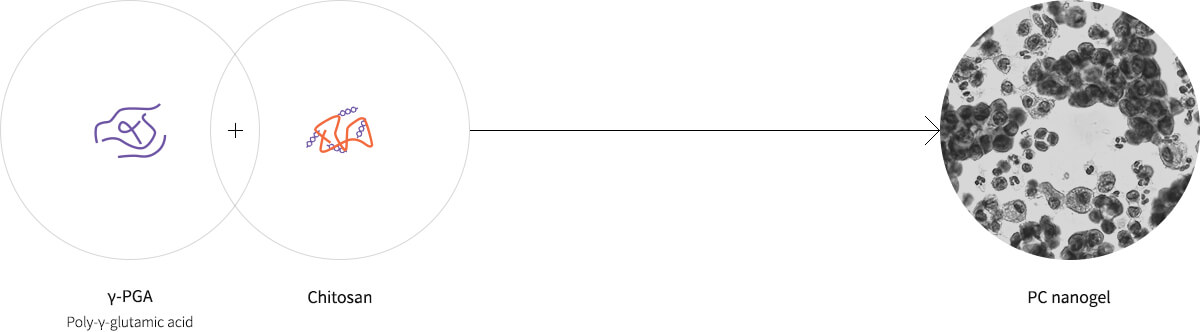
Effects and advantages of adjuvant
Defense against viruses exhibited even from a small
amount of antigens
① Increased antigenicity of vaccine
② Enhanced continuity of immunity
③ Reduced risk of side effects due to antig
Advantages of γ-PGA
Innocuous → Safe with no risk of side effects such as inflammation
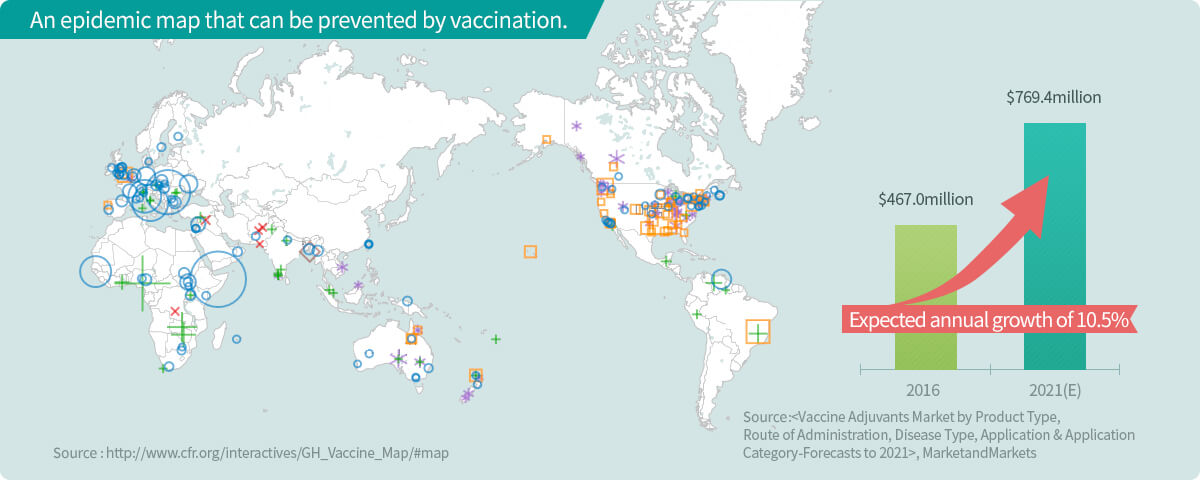
Global market value
More than 30 vaccine adjuvants currently under development both in Korea and worldwide
Status
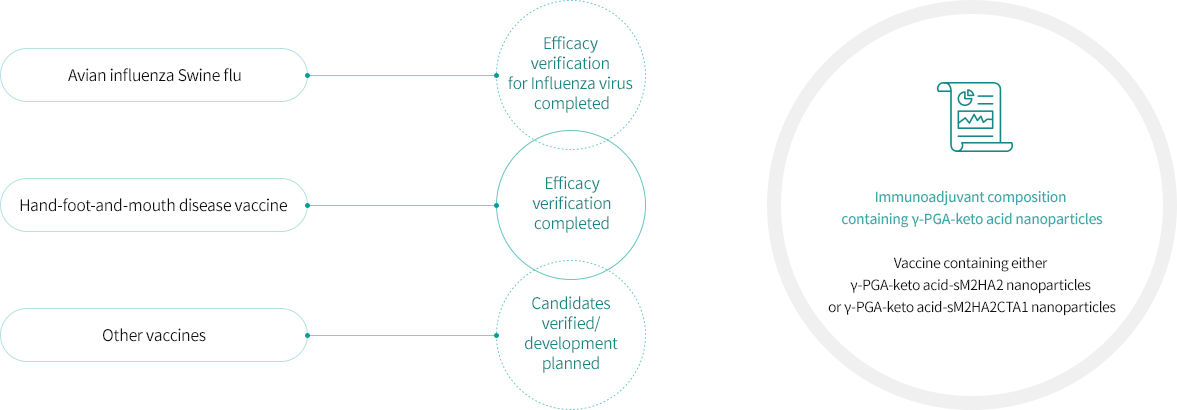
Open innovation among industry, government, and academia
Strategy
